Understanding Exosomes: The Tiny Wonders
What is the deal with this tiny but mighty world of exosomes? These microscopic badboys in the realm of dermatology have been gaining a lot of attention. Medical aesthetics, blending regenerative medicine with cosmetic enhancements, exosomes, cell-secreted vesicles rich in bioactive molecules, are offering great alternatives due to their low response to trigger your body's immune response and strong tissue penetration.
Imagine ultra-small bubbles, barely the width of a human hair, released by almost all types of cells. Inside each of these tiny molecules are proteins, lipids, and genetic materials, including mRNA and miRNA, essential for cellular communication. They're like the body's postal service, delivering crucial messages between cells to maintain healthy skin. Their roles are critical in numerous biological processes such as tissue repair, immune modulation, and even cell differentiation. Simply put, exosomes can play a pivotal role in keeping our skin healthy and vibrant.
Exosomes in Aesthetics: A Beauty Game-Changer
In this industry, exosomes are creating lots of talk like never before. These natural wonders can be infused into topical applications like creams, serums, and masks, working their magic on our skin. How? By delivering nutrients and genetic information that can dramatically enhance skin quality. They stimulate collagen production, essential for youthful skin, combat inflammation, and offer protection against environmental damage. The result? A visible reduction in wrinkles and fine lines, improved skin hydration, and a boost in overall skin health and appearance. Exosomes are paving the way for a new era in beauty, offering a natural, effective solution for ageless beauty. Like I always say, choose a vetted brand and don't fall for good marketing. Ask yourself, does this beauty product really work? What does the clinical data say?

TO THE PROS: Exosomes are administered topically or via local injection in medical aesthetics, with varying effects based on administration mode. For instance, exosomes from mesenchymal stem cells promote wound healing by regulating macrophage behavior, while those from keratinocytes and human amniotic stem cells reduce hyperpigmentation by inhibiting melanin production through targeted microRNA action. Similarly, milk exosomes and those derived from fat mesenchymal stem cells help in controlling hair loss by targeting specific pathways to promote cell proliferation and migration. Additionally, human-induced potent stem cell-derived exosomes have been shown to have anti-aging effects by decreasing the activity of aging markers in cells. The source of exosomes is crucial because it determines the specific microRNAs and proteins they carry, which in turn dictates their therapeutic potential and application in treating various aesthetic concerns, such as wound healing, hyperpigmentation, hair loss, and aging effects. This specificity highlights why understanding and selecting the appropriate source of exosomes is essential for achieving desired outcomes in medical aesthetics. What are some brands? Anteage employs Human Bone Marrow Stem Cell Exosomes and Human Umbilical Cord Stem Cell Exosomes, while Benev utilizes Human Adipose Stromal Cells with a diluent the has a powerful complex of some of my favorite ingredients. That is not to say you can't utilize Anteage's approved transepidermal serums to boost the Exosomes. And Dp Derm. So refer back to the last few sentences and get thinking...
Healing with Exosomes vs GFs vs Stems Cells
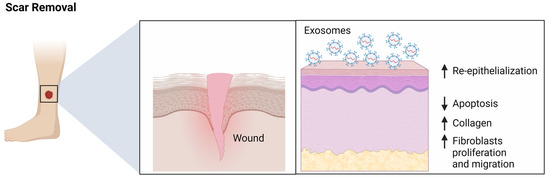
When it comes to healing wounds, exosomes are nothing short of a solid addition. Studies have shown, they can help support the body’s response to injury. They mobilize to the wound site, carrying proteins and genetic materials that kickstart the healing process. By promoting cell-to-cell communication, they help mediate the complex process of wound healing, from reducing infection risk to enhancing tissue regeneration. Particularly in the case of burns and other traumatic skin injuries, exosomes are proving to be invaluable in accelerating recovery and minimizing scarring. So whats the difference between stem cells, exosomes and PRP/PRF? In dermatology, stem cells, exosomes, and Platelet-Rich Plasma/Fibrin (PRP/PRF) are utilized for their unique properties in promoting skin health, rejuvenation, and healing. Their applications in dermatology, though sometimes overlapping, are distinct in mechanism and outcomes.
-
Stem Cells: A
re undifferentiated cells that have the potential to develop into different cell types in the body. They can divide and replicate many times, a process known as proliferation. Focus on regeneration and repair of skin cells. - Application: Stem cells are primarily used for their regenerative and reparative properties. They can differentiate into various types of skin cells and help in repairing damaged skin tissue.
- Uses: In dermatology, stem cell therapy is explored for wound healing, treating burn injuries, and in anti-aging treatments to rejuvenate the skin. Research is also ongoing for using stem cells in treating conditions like alopecia (hair loss).
- Mechanism: Stem cells contribute to the regeneration of skin tissue by differentiating into skin cells and releasing growth factors that promote healing and the formation of new cells.
-
Exosomes: A
re small vesicles (30–200 nanometers in diameter) that are released from cells. They are a type of extracellular vesicle. Act as messengers to enhance communication between cells, influencing repair and rejuvenation processes. - Application: Exosomes are used for their role in cell communication and signaling. They carry proteins, lipids, and genetic material that can influence skin cell behavior.
- Uses: Studies are showing their potential in skin rejuvenation, anti-aging treatments, and in enhancing the healing of skin wounds. Exosomes may be beneficial in cosmetic dermatology for improving skin texture and elasticity.
- Mechanism: Exosomes from certain cell types can promote skin regeneration and healing by delivering specific molecules that stimulate skin cells, modulate immune responses, or promote collagen production. They have low immunogenicity (immune response) and strong tissue penetration
-
Platelet-Rich Plasma/Fibrin (PRP/PRF): A
concentrated form of plasma, a component of blood, containing a high concentration of platelets. Provides a concentration of growth factors and platelets from the patient's own blood, promoting healing and rejuvenation. - Application: PRP/PRF is used for its high concentration of growth factors and platelets that are vital in the healing process.
- Uses: Commonly used in cosmetic dermatology for skin rejuvenation, pigementation, wrinkle reduction, and in the treatment of acne scars. PRP/PRF is also used in hair restoration treatments for conditions like androgenetic alopecia.
- Mechanism: The growth factors in PRP/PRF stimulate cell proliferation, collagen production, and angiogenesis (formation of new blood vessels), which are crucial for skin repair and rejuvenation. PRF will yield less of an immune response due to their being no additives.
Exosomes: Architects of Skin Rejuvenation
Unlike traditional treatments, they leverage the body's natural regenerative capabilities. Through treatments like exosome facial rejuvenation, these vesicles are introduced into the facial skin, potentially reducing signs of aging such as wrinkles, dark spots, and improving skin tone. While still in the research phase, the potential of exosome-based therapies in restoring a youthful, radiant complexion is immense.
Barrier Health & Skin Diseases
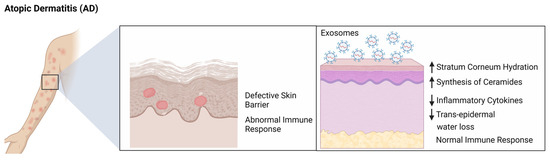
In the battle against challenging skin diseases like Systemic lupus erythematosus (SLE), psoriasis, and atopic dermatitis, exosomes are opening new possibilities. Barrier health is foundational to maintaining overall skin health, with a compromised skin barrier often being at the root of various skin conditions such as eczema, psoriasis, and even acne. The skin barrier functions as a protective shield, guarding against environmental aggressors and maintaining hydration. When this barrier is weakened or damaged, it can lead to increased sensitivity, dryness, and susceptibility to irritants and allergens, exacerbating various skin issues.
Exosomes have emerged as a promising tool in enhancing and restoring the skin barrier function. These tiny vesicles, derived from cellular processes, are packed with proteins, lipids, and nucleic acids that are essential for cellular communication and regeneration. When applied to the skin, exosomes can deliver these crucial biomolecules directly to the skin cells, aiding in repair and strengthening of the skin barrier. Their ability to promote cell-to-cell communication, modulate the immune system and reduce inflammation also helps in coordinating the skin's response to damage and stress, effectively boosting the natural barrier function. This makes exosomes a potential therapeutic agent in treating and managing a wide range of skin conditions linked to impaired barrier health.
Brightening the Future: Exosomes in Pigmentation and Hair Growth
My specialty! Beyond skin health, exosomes help regulate pigmentation and promoting hair growth. This opens up exciting possibilities for treating conditions like post inflammatory hyperpigmentation, melasma, vitiligo, and alopecia, a condition characterized by hair loss.
The Regulatory Perspective
Navigating their regulatory landscape is where we may have challanges. As a relatively new discovery, the FDA are still assessing how to classify and regulate exosome-based treatments. As you know, I always emphasize, not one treatment or product is made the same even if they have the same ingredient. This is a vital step to ensure the safety and efficacy of exosome therapies in dermatology. The ongoing research and regulatory efforts in integrating exosomes safely into mainstream skincare and medical treatments is ongoing!
The world of dermatology and aesthetics continues to develop. Thanks to exosomes, things like healing wounds and treating chronic skin diseases, these tiny particles offer big promises. Do they hold up on that promise? Its too soon to tell longterm but research continues. We can expect more studies, making exosomes a cornerstone of future skin health and beauty therapies. Try our integrative therapeutic treatment that offers a comprehensive rejuvenation with Anteage exosomes!
Reference:
- Alzhrani G. N., Alanazi S. T., Alsharif S. Y., Albalawi A. M., Alsharif A. A., Abdel-Maksoud M. S., et al. (2021). Exosomes: Isolation, characterization, and biomedical applications. Cell. Biol. Int. 45 (9), 1807–1831. 10.1002/cbin.11620 [PubMed] [CrossRef] [Google Scholar]
- An Y., Lin S., Tan X., Zhu S., Nie F., Zhen Y., et al. (2021). Exosomes from adipose-derived stem cells and application to skin wound healing. Cell. Prolif. 54 (3), e12993. 10.1111/cpr.12993 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Arpagaus C., Collenberg A., Rutti D., Assadpour E., Jafari S. M. (2018). Nano spray drying for encapsulation of pharmaceuticals. Int. J. Pharm. X. 546 (1-2), 194–214. 10.1016/j.ijpharm.2018.05.037 [PubMed] [CrossRef] [Google Scholar]
- Atienzar-Aroca S., Flores-Bellver M., Serrano-Heras G., Martinez-Gil N., Barcia J. M., Aparicio S., et al. (2016). Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J. Cell. Mol. Med. 20 (8), 1457–1466. 10.1111/jcmm.12834 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Bae I. S., Kim S. H. (2021). Milk exosome-derived MicroRNA-2478 suppresses melanogenesis through the akt-gsk3β pathway. Cells 10 (11), 2848. 10.3390/cells10112848 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ban J. J., Lee M., Im W., Kim M. (2015). Low pH increases the yield of exosome isolation. Biochem. Biophys. Res. Commun. 461 (1), 76–79. 10.1016/j.bbrc.2015.03.172 [PubMed] [CrossRef] [Google Scholar]
- Barranco I., Padilla L., Parrilla I., Alvarez-Barrientos A., Perez-Patino C., Pena F. J., et al. (2019). Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles. Sci. Rep. 9 (1), 11584. 10.1038/s41598-019-48095-3 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Berckmans R. J., Sturk A., van Tienen L. M., Schaap M. C., Nieuwland R. (2011). Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood 117 (11), 3172–3180. 10.1182/blood-2010-06-290460 [PubMed] [CrossRef] [Google Scholar]
- Boo Y. C. (2022). Metabolic basis and clinical evidence for skin lightening effects of thiol compounds. Antioxidants (Basel) 11 (3), 503. 10.3390/antiox11030503 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Borges F. T., Reis L. A., Schor N. (2013). Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 46 (10), 824–830. 10.1590/1414-431X20132964 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Bosch S., de Beaurepaire L., Allard M., Mosser M., Heichette C., Chretien D., et al. (2016). Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 6, 36162. 10.1038/srep36162 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Budgude P., Kale V., Vaidya A. (2021). Cryopreservation of mesenchymal stromal cell-derived extracellular vesicles using trehalose maintains their ability to expand hematopoietic stem cells in vitro . Cryobiology 98, 152–163. 10.1016/j.cryobiol.2020.11.009 [PubMed] [CrossRef] [Google Scholar]
- Busatto S., Vilanilam G., Ticer T., Lin W. L., Dickson D. W., Shapiro S., et al. (2018). Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells 7 (12), 273. 10.3390/cells7120273 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cao J., Wang B., Tang T., Lv L., Ding Z., Li Z., et al. (2020). Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell. Res. Ther. 11 (1), 206. 10.1186/s13287-020-01719-2 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cao L., Tian T., Huang Y., Tao S., Zhu X., Yang M., et al. (2021). Neural progenitor cell-derived nanovesicles promote hair follicle growth via miR-100. J. Nanobiotechnology 19 (1), 20. 10.1186/s12951-020-00757-5 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Cha J. M., Shin E. K., Sung J. H., Moon G. J., Kim E. H., Cho Y. H., et al. (2018). Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci. Rep. 8 (1), 1171. 10.1038/s41598-018-19211-6 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Chamberlain C. S., Kink J. A., Wildenauer L. A., McCaughey M., Henry K., Spiker A. M., et al. (2021). Exosome-educated macrophages and exosomes differentially improve ligament healing. Stem Cells 39 (1), 55–61. 10.1002/stem.3291[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Chen J., Zhou R., Liang Y., Fu X., Wang D., Wang C. (2019). Blockade of lncRNA-ASLNCS5088-enriched exosome generation in M2 macrophages by GW4869 dampens the effect of M2 macrophages on orchestrating fibroblast activation. FASEB J. 33 (11), 12200–12212. 10.1096/fj.201901610 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Chen T. S., Arslan F., Yin Y., Tan S. S., Lai R. C., Choo A. B., et al. (2011). Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J. Transl. Med. 9, 47. 10.1186/1479-5876-9-47 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Chen Y., Zhu Q., Cheng L., Wang Y., Li M., Yang Q., et al. (2021). Exosome detection via the ultrafast-isolation system: Exodus. Nat. Methods 18 (2), 212–218. 10.1038/s41592-020-01034-x [PubMed] [CrossRef] [Google Scholar]
- Cheng J., Zhao Z. W., Wen J. R., Wang L., Huang L. W., Yang Y. L., et al. (2020). Status, challenges, and future prospects of stem cell therapy in pelvic floor disorders. World J. Clin. Cases 8 (8), 1400–1413. 10.12998/wjcc.v8.i8.1400 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cheng Y., Zeng Q., Han Q., Xia W. (2019). Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell. 10 (4), 295–299. 10.1007/s13238-018-0529-4 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Choi J. S., Cho W. L., Choi Y. J., Kim J. D., Park H. A., Kim S. Y., et al. (2019). Functional recovery in photo-damaged human dermal fibroblasts by human adipose-derived stem cell extracellular vesicles. J. Extracell. Vesicles 8 (1), 1565885. 10.1080/20013078.2019.1565885 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Clement J., Wong M., Poljak A., Sachdev P., Braidy N. (2019). The plasma NAD(+) metabolome is dysregulated in "normal" aging. Rejuvenation Res. 22 (2), 121–130. 10.1089/rej.2018.2077 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cocozza F., Menay F., Tsacalian R., Elisei A., Sampedro P., Soria I., et al. (2019). Cyclophosphamide enhances the release of tumor exosomes that elicit a specific immune response in vivo in a murine T-cell lymphoma. Vaccine 37 (12), 1565–1576. 10.1016/j.vaccine.2019.02.004 [PubMed] [CrossRef] [Google Scholar]
- Crawford S., Diamond D., Brustolon L., Penarreta R. (2010). Effect of increased extracellular Ca++ on microvesicle production and tumor spheroid formation. Cancer Microenviron. 4 (1), 93–103. 10.1007/s12307-010-0049-0 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Dad H. A., Gu T. W., Zhu A. Q., Huang L. Q., Peng L. H. (2021). Plant exosome-like nanovesicles: Emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. 29 (1), 13–31. 10.1016/j.ymthe.2020.11.030 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Das A., Mohan V., Krishnaswamy V. R., Solomonov I., Sagi I. (2019). Exosomes as a storehouse of tissue remodeling proteases and mediators of cancer progression. Cancer Metastasis Rev. 38 (3), 455–468. 10.1007/s10555-019-09813-5 [PubMed] [CrossRef] [Google Scholar]
- Domenis R., Zanutel R., Caponnetto F., Toffoletto B., Cifu A., Pistis C., et al. (2017). Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediat. Inflamm. 2017, 1–11. 10.1155/2017/4814987 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Edmonds A. (2013). Can medicine be aesthetic? Disentangling beauty and health in elective surgeries. Med. Anthropol. Q.27 (2), 233–252. 10.1111/maq.12025 [PubMed] [CrossRef] [Google Scholar]
- Egger A., Tomic-Canic M., Tosti A. (2020). Advances in stem cell-based therapy for hair loss. CellR4. Repair Replace. Regen. Reprogr. 8, e2894. [PMC free article] [PubMed] [Google Scholar]
- Ekström E. J., Bergenfelz C., von Bülow V., Serifler F., Carlemalm E., Jönsson G., et al. (2014). WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol. Cancer 13, 88. 10.1186/1476-4598-13-88 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Foo J. B., Looi Q. H., How C. W., Lee S. H., Al-Masawa M. E., Chong P. P., et al. (2021). Mesenchymal stem cell-derived exosomes and MicroRNAs in cartilage regeneration: Biogenesis, efficacy, miRNA enrichment and delivery. Pharm. (Basel) 14 (11), 1093. 10.3390/ph14111093 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Gao W., Wang X., Si Y., Pang J., Liu H., Li S., et al. (2021). Exosome derived from ADSCs attenuates ultraviolet B-mediated photoaging in human dermal fibroblasts. Photochem. Photobiol. 97 (4), 795–804. 10.1111/php.13370 [PubMed] [CrossRef] [Google Scholar]
- Garcia N. A., Ontoria-Oviedo I., Gonzalez-King H., Diez-Juan A., Sepulveda P. (2015). Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One 10 (9), e0138849. 10.1371/journal.pone.0138849 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Gartz M., Darlington A., Afzal M. Z., Strande J. L. (2018). Exosomes exert cardioprotection in dystrophin-deficient cardiomyocytes via ERK1/2-p38/MAPK signaling. Sci. Rep. 8 (1), 16519. 10.1038/s41598-018-34879-6 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Gazitaeva Z. I., Drobintseva A. O., Prokopov A. Y., Sidorina A. N., Leonteva D. O., Kvetnoy I. M. (2021). Signaling molecules of human skin cells as the targets for injection cosmetology. Clin. Cosmet. Investig. Dermatol. 14, 1473–1480. 10.2147/CCID.S321104 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Geng H. Y., Feng Z. J., Zhang J. J., Li G. Y. (2021). Exosomal CLIC1 released by CLL promotes HUVECs angiogenesis by regulating ITGβ1‐MAPK/ERK axis. Kaohsiung J. Med. Sci. 37 (3), 226–235. 10.1002/kjm2.12287 [PubMed] [CrossRef] [Google Scholar]
- Gentile P., Garcovich S. (2019). Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: Wnt pathway, growth-factor, and mesenchymal stem cell signaling impact analysis on cell growth and hair follicle development. Cells 8 (5), 466. 10.3390/cells8050466 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Gimona M., Pachler K., Laner-Plamberger S., Schallmoser K., Rohde E. (2017). Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int. J. Mol. Sci. 18 (6), 1190. 10.3390/ijms18061190 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Golchin A. (2021). Cell-based therapy for severe COVID-19 patients: Clinical trials and cost-utility. Stem Cell. Rev. Rep.17 (1), 56–62. 10.1007/s12015-020-10046-1 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Guix F. X., Sannerud R., Berditchevski F., Arranz A. M., Horre K., Snellinx A., et al. (2017). Tetraspanin 6: A pivotal protein of the multiple vesicular body determining exosome release and lysosomal degradation of amyloid precursor protein fragments. Mol. Neurodegener. 12 (1), 25. 10.1186/s13024-017-0165-0 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Gurunathan S., Kang M. H., Qasim M., Khan K., Kim J. H. (2021). Biogenesis, membrane trafficking, functions, and next generation nanotherapeutics medicine of extracellular vesicles. Int. J. Nanomedicine 16, 3357–3383. 10.2147/IJN.S310357[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ha D. H., Kim H. K., Lee J., Kwon H. H., Park G. H., Yang S. H., et al. (2020a). Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells 9 (5), 1157. 10.3390/cells9051157 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ha D. H., Kim S. D., Lee J., Kwon H. H., Park G. H., Yang S. H., et al. (2020b). Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul. Toxicol. Pharmacol. 115, 104686. 10.1016/j.yrtph.2020.104686 [PubMed] [CrossRef] [Google Scholar]
- Ha D., Yang N., Nadithe V. (2016). Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 6 (4), 287–296. 10.1016/j.apsb.2016.02.001[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hade M. D., Suire C. N., Suo Z. (2021). Mesenchymal stem cell-derived exosomes: Applications in regenerative medicine. Cells 10 (8), 1959. 10.3390/cells10081959 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hakozaki T., Minwalla L., Zhuang J., Chhoa M., Matsubara A., Miyamoto K., et al. (2002). The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 147 (1), 20–31. 10.1046/j.1365-2133.2002.04834.x [PubMed] [CrossRef] [Google Scholar]
- Han G., Kim H., Kim D. E., Ahn Y., Kim J., Jang Y. J., et al. (2022). The potential of bovine colostrum-derived exosomes to repair aged and damaged skin cells. Pharmaceutics 14 (2), 307. 10.3390/pharmaceutics14020307 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Han X., Wu P., Li L., Sahal H. M., Ji C., Zhang J., et al. (2021). Exosomes derived from autologous dermal fibroblasts promote diabetic cutaneous wound healing through the Akt/β-catenin pathway. Cell. Cycle 20 (5-6), 616–629. 10.1080/15384101.2021.1894813 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hansen L. J. J., Daoussi R., Vervaet C., Remon J. P., De Beer T. R. M. (2015). Freeze-drying of live virus vaccines: A review. Vaccine 33 (42), 5507–5519. 10.1016/j.vaccine.2015.08.085 [PubMed] [CrossRef] [Google Scholar]
- Haraszti R. A., Miller R., Stoppato M., Sere Y. Y., Coles A., Didiot M. C., et al. (2018). Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 26 (12), 2838–2847. 10.1016/j.ymthe.2018.09.015 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- He F., Liu H., Guo X., Yin B. C., Ye B. C. (2017). Direct exosome quantification via bivalent-cholesterol-labeled DNA anchor for signal amplification. Anal. Chem. 89 (23), 12968–12975. 10.1021/acs.analchem.7b03919 [PubMed] [CrossRef] [Google Scholar]
- He X., Dong Z., Cao Y., Wang H., Liu S., Liao L., et al. (2019). MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019, 1–16. 10.1155/2019/7132708 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hettich B. F., Ben-Yehuda Greenwald M., Werner S., Leroux J. C. (2020). Exosomes for wound healing: Purification optimization and identification of bioactive components. Adv. Sci. (Weinh). 7 (23), 2002596. 10.1002/advs.202002596[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hong C., Zhang G. L., Zhang W., Liu J. Q., Zhang J., Chen Y. T., et al. (2021). Hair grows hair: Dual-effective hair regrowth through a hair enhanced dissolvable microneedle patch cooperated with the pure yellow light irradiation. Appl. Mater. Today 25, 101188. 10.1016/j.apmt.2021.101188 [CrossRef] [Google Scholar]
- Hu S., Li Z., Cores J., Huang K., Su T., Dinh P. U., et al. (2019). Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano 13 (10), 11273–11282. 10.1021/acsnano.9b04384[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Huang J., Xiong J., Yang L., Zhang J., Sun S., Liang Y. (2021). Cell-free exosome-laden scaffolds for tissue repair. Nanoscale 13 (19), 8740–8750. 10.1039/d1nr01314a [PubMed] [CrossRef] [Google Scholar]
- Hurwitz S. N., Nkosi D., Conlon M. M., York S. B., Liu X., Tremblay D. C., et al. (2017). CD63 regulates epstein-barr virus LMP1 exosomal packaging, enhancement of vesicle production, and noncanonical NF-κB signaling. J. Virol. 91, e02251. 10.1128/JVI.02251-16 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hwang I., Hong S. (2017). Neural stem cells and its derivatives as a new material for melanin inhibition. Int. J. Mol. Sci.19 (1), 36. 10.3390/ijms19010036 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Jackson W. M., Nesti L. J., Tuan R. S. (2012). Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl. Med. 1 (1), 44–50. 10.5966/sctm.2011-0024 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Jafari D., Malih S., Eini M., Jafari R., Gholipourmalekabadi M., Sadeghizadeh M., et al. (2020). Improvement, scaling-up, and downstream analysis of exosome production. Crit. Rev. Biotechnol. 40 (8), 1098–1112. 10.1080/07388551.2020.1805406 [PubMed] [CrossRef] [Google Scholar]
- Jing H., He X., Zheng J. (2018). Exosomes and regenerative medicine: State of the art and perspectives. Transl. Res. 196, 1–16. 10.1016/j.trsl.2018.01.005 [PubMed] [CrossRef] [Google Scholar]
- Johari B., Asadi Z., Rismani E., Maghsood F., Sheikh Rezaei Z., Farahani S., et al. (2019). Inhibition of transcription factor T-cell factor 3 (TCF3) using the oligodeoxynucleotide strategy increases embryonic stem cell stemness: Possible application in regenerative medicine. Cell. Biol. Int. 43 (8), 852–862. 10.1002/cbin.11153 [PubMed] [CrossRef] [Google Scholar]
- Kalluri R., LeBleu V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367 (6478), eaau6977. 10.1126/science.aau6977 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Keklikoglou I., Cianciaruso C., Guc E., Squadrito M. L., Spring L. M., Tazzyman S., et al. (2019). Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat. Cell. Biol. 21 (2), 190–202. 10.1038/s41556-018-0256-3[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kim E. S., Jeon H. B., Lim H., Shin J. H., Park S. J., Jo Y. K., et al. (2015). Conditioned media from human umbilical cord blood-derived mesenchymal stem cells inhibits melanogenesis by promoting proteasomal degradation of MITF. PLoS One10 (5), e0128078. 10.1371/journal.pone.0128078 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kim M., Park J. H. (2022). Isolation of aloe saponaria-derived extracellular vesicles and investigation of their potential for chronic wound healing. Pharmaceutics 14 (9), 1905. 10.3390/pharmaceutics14091905 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kim S. R., Zou X., Tang H., Puranik A. S., Abumoawad A. M., Zhu X. Y., et al. (2021). Increased cellular senescence in the murine and human stenotic kidney: Effect of mesenchymal stem cells. J. Cell. Physiol. 236 (2), 1332–1344. 10.1002/jcp.29940 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- King H. W., Michael M. Z., Gleadle J. M. (2012). Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 12, 421. 10.1186/1471-2407-12-421 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kirkland J. L., Tchkonia T. (2017). Cellular senescence: A translational perspective. EBioMedicine 21, 21–28. 10.1016/j.ebiom.2017.04.013 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kojima R., Bojar D., Rizzi G., Hamri G. C., El-Baba M. D., Saxena P., et al. (2018). Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson's disease treatment. Nat. Commun. 9 (1), 1305. 10.1038/s41467-018-03733-8 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kost Y., Muskat A., Mhaimeed N., Nazarian R. S., Kobets K. (2022). Exosome therapy in hair regeneration: A literature review of the evidence, challenges, and future opportunities. J. Cosmet. Dermatol. 21, 3226–3231. 10.1111/jocd.15008 [PubMed] [CrossRef] [Google Scholar]
- Kusuma G. D., Barabadi M., Tan J. L., Morton D. A. V., Frith J. E., Lim R. (2018). To protect and to preserve: Novel preservation strategies for extracellular vesicles. Front. Pharmacol. 9, 1199. 10.3389/fphar.2018.01199 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Lee H., Cha H., Park J. H. (2020a). Derivation of cell-engineered nanovesicles from human induced pluripotent stem cells and their protective effect on the senescence of dermal fibroblasts. Int. J. Mol. Sci. 21 (1), 343. 10.3390/ijms21010343[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lee J. H., Ha D. H., Go H. K., Youn J., Kim H. K., Jin R. C., et al. (2020c). Reproducible large-scale isolation of exosomes from adipose tissue-derived mesenchymal stem/stromal cells and their application in acute kidney injury. Int. J. Mol. Sci.21 (13), 4774. 10.3390/ijms21134774 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lee J. H., Yoon J. Y., Lee J. H., Lee H. H., Knowles J. C., Kim H. W. (2021). Emerging biogenesis technologies of extracellular vesicles for tissue regenerative therapeutics. J. Tissue Eng. 12, 204173142110190. 10.1177/20417314211019015 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lee J., Seok J. M., Huh S. J., Byun H., Lee S., Park S. A., et al. (2020b). 3D printed micro-chambers carrying stem cell spheroids and pro-proliferative growth factors for bone tissue regeneration. Biofabrication 13 (1), 015011. 10.1088/1758-5090/abc39c [PubMed] [CrossRef] [Google Scholar]
- Lee Y., El Andaloussi S., Wood M. J. (2012). Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 21 (R1), R125–R134. 10.1093/hmg/dds317 [PubMed] [CrossRef] [Google Scholar]
- Leggio L., Arrabito G., Ferrara V., Vivarelli S., Paternò G., Marchetti B., et al. (2020). Mastering the tools: Natural versus artificial vesicles in nanomedicine. Adv. Healthc. Mat. 9 (18), e2000731. 10.1002/adhm.202000731 [PubMed] [CrossRef] [Google Scholar]
- Lener T., Gimona M., Aigner L., Borger V., Buzas E., Camussi G., et al. (2015). Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J. Extracell. Vesicles 4, 30087. 10.3402/jev.v4.30087 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Li D., Wu N. (2022). Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res. Clin. Pract. 187, 109882. 10.1016/j.diabres.2022.109882 [PubMed] [CrossRef] [Google Scholar]
- Li L., Zhang Y., Mu J., Chen J., Zhang C., Cao H., et al. (2020a). Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 20 (6), 4298–4305. 10.1021/acs.nanolett.0c00929 [PubMed] [CrossRef] [Google Scholar]
- Li M., Li S., Du C., Zhang Y., Li Y., Chu L., et al. (2020b). Exosomes from different cells: Characteristics, modifications, and therapeutic applications. Eur. J. Med. Chem. 207, 112784. 10.1016/j.ejmech.2020.112784 [PubMed] [CrossRef] [Google Scholar]
- Li W., Huang X., Yu W., Xu Y., Huang R., Park J., et al. (2022a). Activation of functional somatic stem cells promotes endogenous tissue regeneration. J. Dent. Res. 101 (7), 802–811. 10.1177/00220345211070222 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Li X., He X., Wang J., Wang D., Cong P., Zhu A., et al. (2020c). The regulation of exosome-derived miRNA on heterogeneity of macrophages in atherosclerotic plaques. Front. Immunol. 11, 2175. 10.3389/fimmu.2020.02175 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Li Y., Wang G., Wang Q., Zhang Y., Cui L., Huang X. (2022b). Exosomes secreted from adipose-derived stem cells are a potential treatment agent for immune-mediated alopecia. J. Immunol. Res. 2022, 1–14. 10.1155/2022/7471246 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Li Y., Xiao Q., Tang J., Xiong L., Li L. (2021a). Extracellular vesicles: Emerging therapeutics in cutaneous lesions. Int. J. Nanomedicine 16, 6183–6202. 10.2147/ijn.S322356 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Li Y., Zhang J., Shi J., Liu K., Wang X., Jia Y., et al. (2021b). Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell. Res. Ther. 12 (1), 221. 10.1186/s13287-021-02290-0 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Li Z. Q., Kong L., Liu C., Xu H. G. (2020d). Human bone marrow mesenchymal stem cell-derived exosomes attenuate IL-1β-induced annulus fibrosus cell damage. Am. J. Med. Sci. 360 (6), 693–700. 10.1016/j.amjms.2020.07.025 [PubMed] [CrossRef] [Google Scholar]
- Liang Y., Duan L., Lu J., Xia J. (2021). Engineering exosomes for targeted drug delivery. Theranostics 11 (7), 3183–3195. 10.7150/thno.52570 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Liao C. M., Luo T., von der Ohe J., de Juan Mora B., Schmitt R., Hass R. (2021). Human MSC-derived exosomes reduce cellular senescence in renal epithelial cells. Int. J. Mol. Sci. 22 (24), 13562. 10.3390/ijms222413562 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Liu S. J., Meng M. Y., Han S., Gao H., Zhao Y. Y., Yang Y., et al. (2021). Umbilical cord mesenchymal stem cell-derived exosomes ameliorate HaCaT cell photo-aging. Rejuvenation Res. 24 (4), 283–293. 10.1089/rej.2020.2313 [PubMed] [CrossRef] [Google Scholar]
- Liu Y., Xue L., Gao H., Chang L., Yu X., Zhu Z., et al. (2019). Exosomal miRNA derived from keratinocytes regulates pigmentation in melanocytes. J. Dermatol. Sci. 93 (3), 159–167. 10.1016/j.jdermsci.2019.02.001 [PubMed] [CrossRef] [Google Scholar]
- Livshits M. A., Khomyakova E., Evtushenko E. G., Lazarev V. N., Kulemin N. A., Semina S. E., et al. (2015). Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 5, 17319. 10.1038/srep17319 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lo Cicero A., Delevoye C., Gilles-Marsens F., Loew D., Dingli F., Guere C., et al. (2015). Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 6, 7506. 10.1038/ncomms8506 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Lobb R. J., Becker M., Wen S. W., Wong C. S., Wiegmans A. P., Leimgruber A., et al. (2015). Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 4, 27031. 10.3402/jev.v4.27031[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lu M., Peng L., Ming X., Wang X., Cui A., Li Y., et al. (2019). Enhanced wound healing promotion by immune response-free monkey autologous iPSCs and exosomes vs. their allogeneic counterparts. EBioMedicine 42, 443–457. 10.1016/j.ebiom.2019.03.011 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ma L., Chen C., Liu D., Huang Z., Li J., Liu H., et al. (2023). Apoptotic extracellular vesicles are metabolized regulators nurturing the skin and hair. Bioact. Mat. 19, 626–641. 10.1016/j.bioactmat.2022.04.022 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ma T., Fu B., Yang X., Xiao Y., Pan M. (2019). Adipose mesenchymal stem cell‐derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β‐catenin signaling in cutaneous wound healing. J. Cell. Biochem. 120 (6), 10847–10854. 10.1002/jcb.28376 [PubMed] [CrossRef] [Google Scholar]
- Mahdavinezhad F., Gilani M. A. S., Gharaei R., Ashrafnezhad Z., Valipour J., Nashtaei M. S., et al. (2022). Protective roles of seminal plasma exosomes and microvesicles during human sperm cryopreservation. Reprod. Biomed. Online 45 (2), 341–353. 10.1016/j.rbmo.2022.03.033 [PubMed] [CrossRef] [Google Scholar]
- Manconi M., Manca M. L., Marongiu F., Caddeo C., Castangia I., Petretto G. L., et al. (2016). Chemical characterization of Citrus limon var. pompia and incorporation in phospholipid vesicles for skin delivery. Int. J. Pharm. X. 506 (1-2), 449–457. 10.1016/j.ijpharm.2016.04.014 [PubMed] [CrossRef] [Google Scholar]
- Marcus M. E., Leonard J. N. (2013). FedExosomes: Engineering therapeutic biological nanoparticles that truly deliver. Pharm. (Basel) 6 (5), 659–680. 10.3390/ph6050659 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Marino J., Babiker-Mohamed M. H., Crosby-Bertorini P., Paster J. T., LeGuern C., Germana S., et al. (2016). Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci. Immunol. 1 (1), aaf8759. 10.1126/sciimmunol.aaf8759 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Marofi F., Alexandrovna K. I., Margiana R., Bahramali M., Suksatan W., Abdelbasset W. K., et al. (2021). MSCs and their exosomes: A rapidly evolving approach in the context of cutaneous wounds therapy. Stem Cell. Res. Ther. 12 (1), 597. 10.1186/s13287-021-02662-6 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Maroto R., Zhao Y., Jamaluddin M., Popov V. L., Wang H., Kalubowilage M., et al. (2017). Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles 6 (1), 1359478. 10.1080/20013078.2017.1359478 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Martínez-González M. C., Martínez-González R. A., Guerra-Tapia A. (2019). Key communication skills in cosmetic dermatology: A 3-pillar model. Actas Sifiliogr. 110 (10), 794–799. 10.1016/j.adengl.2019.10.003 [PubMed] [CrossRef] [Google Scholar]
- Matic S., D'Souza D. H., Wu T., Pangloli P., Dia V. P. (2020). Bovine milk exosomes affect proliferation and protect macrophages against cisplatin-induced cytotoxicity. Immunol. Invest. 49 (7), 711–725. 10.1080/08820139.2020.1769647 [PubMed] [CrossRef] [Google Scholar]
- Mazini L., Rochette L., Hamdan Y., Malka G. (2021). Skin immunomodulation during regeneration: Emerging new targets. J. Pers. Med. 11 (2), 85. 10.3390/jpm11020085 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- McReynolds M. R., Chellappa K., Baur J. A. (2020). Age-related NAD(+) decline. Exp. Gerontol. 134, 110888. 10.1016/j.exger.2020.110888 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- McReynolds M. R., Chellappa K., Chiles E., Jankowski C., Shen Y., Chen L., et al. (2021). NAD(+) flux is maintained in aged mice despite lower tissue concentrations. Cell. Syst. 12 (12), 1160–1172.e4. e1164. 10.1016/j.cels.2021.09.001 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Mehryab F., Rabbani S., Shahhosseini S., Shekari F., Fatahi Y., Baharvand H., et al. (2020). Exosomes as a next-generation drug delivery system: An update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 113, 42–62. 10.1016/j.actbio.2020.06.036 [PubMed] [CrossRef] [Google Scholar]
- Mol E. A., Goumans M. J., Doevendans P. A., Sluijter J. P. G., Vader P. (2017). Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine Nanotechnol. Biol. Med. 13 (6), 2061–2065. 10.1016/j.nano.2017.03.011 [PubMed] [CrossRef] [Google Scholar]
- Muramatsu H., Lam K., Bajusz C., Laczkó D., Karikó K., Schreiner P., et al. (2022). Lyophilization provides long-term stability for a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine. Mol. Ther. 30 (5), 1941–1951. 10.1016/j.ymthe.2022.02.001 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Naik P. P. (2021). Utilities of botulinum toxins in dermatology and cosmetology. Clin. Cosmet. Investig. Dermatol. 14, 1319–1330. 10.2147/CCID.S332247 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Nakanishi K., Tomita M., Tsumoto K. (2020). Membrane fusion and infection abilities of baculovirus virions are preserved during freezing and thawing in the presence of trehalose. Biosci. Biotechnol. Biochem. 84 (4), 686–694. 10.1080/09168451.2019.1704396 [PubMed] [CrossRef] [Google Scholar]
- Nam G. H., Choi Y., Kim G. B., Kim S., Kim S. A., Kim I. S. (2020). Emerging prospects of exosomes for cancer treatment: From conventional therapy to immunotherapy. Adv. Mat. 32 (51), e2002440. 10.1002/adma.202002440 [PubMed] [CrossRef] [Google Scholar]
- Nilforoushzadeh M. A., Aghdami N., Taghiabadi E. (2020). Human hair outer root sheath cells and platelet-lysis exosomes promote hair inductivity of dermal papilla cell. Tissue Eng. Regen. Med. 17 (4), 525–536. 10.1007/s13770-020-00266-4[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Nordin J. Z., Lee Y., Vader P., Mager I., Johansson H. J., Heusermann W., et al. (2015). Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine Nanotechnol. Biol. Med. 11 (4), 879–883. 10.1016/j.nano.2015.01.003 [PubMed] [CrossRef] [Google Scholar]
- O'Brien K., Breyne K., Ughetto S., Laurent L. C., Breakefield X. O. (2020). RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell. Biol. 21 (10), 585–606. 10.1038/s41580-020-0251-y [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Odrobinska J., Mielanczyk L., Neugebauer D. (2020). 4-n-Butylresorcinol-Based linear and graft polymethacrylates for arbutin and vitamins delivery by micellar systems. Polym. (Basel) 12 (2), 330. 10.3390/polym12020330 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Ogawa R. (2017). Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int. J. Mol. Sci. 18 (3), 606. 10.3390/ijms18030606 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Oh M., Lee J., Kim Y. J., Rhee W. J., Park J. H. (2018). Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 19 (6), 1715. 10.3390/ijms19061715 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Pegtel D. M., Gould S. J. (2019). Exosomes. Annu. Rev. Biochem. 88, 487–514. 10.1146/annurev-biochem-013118-111902 [PubMed] [CrossRef] [Google Scholar]
- Peña-Juárez M. C., Guadarrama-Escobar O. R., Escobar-Chávez J. J. (2022). Transdermal delivery systems for biomolecules. J. Pharm. Innov. 17 (2), 319–332. 10.1007/s12247-020-09525-2 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Petersen K. E., Shiri F., White T., Bardi G. T., Sant H., Gale B. K., et al. (2018). Exosome isolation: Cyclical electrical field flow fractionation in low-ionic-strength fluids. Anal. Chem. 90 (21), 12783–12790. 10.1021/acs.analchem.8b03146 [PubMed] [CrossRef] [Google Scholar]
- Potter M., Lins B., Mietzsch M., Heilbronn R., Van Vliet K., Chipman P., et al. (2014). A simplified purification protocol for recombinant adeno-associated virus vectors. Mol. Ther. - Methods & Clin. Dev. 1, 14034. 10.1038/mtm.2014.34 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Prasai A., Jay J. W., Jupiter D., Wolf S. E., El Ayadi A. (2022). Role of exosomes in dermal wound healing: A systematic review. J. Invest. Dermatol. 142, 662–678.e8. e668. 10.1016/j.jid.2021.07.167 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Qi J., Liu Q., Reisdorf R. L., Boroumand S., Behfar A., Moran S. L., et al. (2020). Characterization of a purified exosome product and its effects on canine flexor tenocyte biology. J. Orthop. Res. 38 (8), 1845–1855. 10.1002/jor.24587 [PubMed] [CrossRef] [Google Scholar]
- Rajendran R. L., Gangadaran P., Bak S. S., Oh J. M., Kalimuthu S., Lee H. W., et al. (2017). Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci. Rep. 7 (1), 15560. 10.1038/s41598-017-15505-3 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Rao D., Huang D., Sang C., Zhong T., Zhang Z., Tang Z. (2021). Advances in mesenchymal stem cell-derived exosomes as drug delivery vehicles. Front. Bioeng. Biotechnol. 9, 797359. 10.3389/fbioe.2021.797359 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ras-Carmona A., Gomez-Perosanz M., Reche P. A. (2021). Prediction of unconventional protein secretion by exosomes. BMC Bioinforma. 22 (1), 333. 10.1186/s12859-021-04219-z [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ratz-Lyko A., Arct J. (2019). Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 21 (2), 84–90. 10.1080/14764172.2018.1469767 [PubMed] [CrossRef] [Google Scholar]
- Regimbeau M., Abrey J., Vautrot V., Causse S., Gobbo J., Garrido C. (2021). Heat shock proteins and exosomes in cancer theranostics. Semin. Cancer Biol. 86, 46–57. 10.1016/j.semcancer.2021.07.014 [PubMed] [CrossRef] [Google Scholar]
- Reiner A. T., Witwer K. W., van Balkom B. W. M., de Beer J., Brodie C., Corteling R. L., et al. (2017). Concise review: Developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl. Med. 6 (8), 1730–1739. 10.1002/sctm.17-0055 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Riau A. K., Ong H. S., Yam G. H. F., Mehta J. S. (2019). Sustained delivery system for stem cell-derived exosomes. Front. Pharmacol. 10, 1368. 10.3389/fphar.2019.01368 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Saadeldin I. M., Khalil W. A., Alharbi M. G., Lee S. H. (2020). The current trends in using nanoparticles, liposomes, and exosomes for semen cryopreservation. Anim. (Basel) 10 (12), 2281. 10.3390/ani10122281 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Sahin F., Kocak P., Gunes M. Y., Ozkan I., Yildirim E., Kala E. Y. (2019). In vitro wound healing activity of wheat-derived nanovesicles. Appl. Biochem. Biotechnol. 188 (2), 381–394. 10.1007/s12010-018-2913-1 [PubMed] [CrossRef] [Google Scholar]
- Salimu J., Webber J., Gurney M., Al-Taei S., Clayton A., Tabi Z. (2017). Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J. Extracell. Vesicles 6 (1), 1368823. 10.1080/20013078.2017.1368823[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Savcı Y., Kırbaş O. K., Bozkurt B. T., Abdik E. A., Taşlı P. N., Şahin F., et al. (2021). Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 12 (11), 5144–5156. 10.1039/d0fo02953j [PubMed] [CrossRef] [Google Scholar]
- Schiller L. T., Lemus-Diaz N., Rinaldi Ferreira R., Boker K. O., Gruber J. (2018). Enhanced production of exosome-associated AAV by overexpression of the tetraspanin CD9. Mol. Ther. - Methods & Clin. Dev. 9, 278–287. 10.1016/j.omtm.2018.03.008 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Shabbir A., Cox A., Rodriguez-Menocal L., Salgado M., Van Badiavas E. (2015). Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro . Stem Cells Dev.24 (14), 1635–1647. 10.1089/scd.2014.0316 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Shang H., Younas A., Zhang N. (2022). Recent advances on transdermal delivery systems for the treatment of arthritic injuries: From classical treatment to nanomedicines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 14 (3), e1778. 10.1002/wnan.1778 [PubMed] [CrossRef] [Google Scholar]
- Shekhter A. B., Fayzullin A. L., Vukolova M. N., Rudenko T. G., Osipycheva V. D., Litvitsky P. F. (2019). Medical applications of collagen and collagen-based materials. Curr. Med. Chem. 26 (3), 506–516. 10.2174/0929867325666171205170339 [PubMed] [CrossRef] [Google Scholar]
- Shi H., Wang M., Sun Y., Yang D., Xu W., Qian H. (2021). Exosomes: Emerging cell-free based therapeutics in dermatologic diseases. Front. Cell. Dev. Biol. 9, 736022. 10.3389/fcell.2021.736022 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Shiekh P. A., Singh A., Kumar A. (2020). Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 249, 120020. 10.1016/j.biomaterials.2020.120020 [PubMed] [CrossRef] [Google Scholar]
- Shin K. O., Ha D. H., Kim J. O., Crumrine D. A., Meyer J. M., Wakefield J. S., et al. (2020). Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de Novo synthesis of ceramides in atopic dermatitis. Cells 9 (3), 680. 10.3390/cells9030680 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Shinde P., Khan N., Melinkeri S., Kale V., Limaye L. (2019). Freezing of dendritic cells with trehalose as an additive in the conventional freezing medium results in improved recovery after cryopreservation. Transfusion 59 (2), 686–696. 10.1111/trf.15028 [PubMed] [CrossRef] [Google Scholar]
- Sinha S., Hoshino D., Hong N. H., Kirkbride K. C., Grega-Larson N. E., Seiki M., et al. (2016). Cortactin promotes exosome secretion by controlling branched actin dynamics. J. Cell. Biol. 214 (2), 197–213. 10.1083/jcb.201601025 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Taghiabadi E., Nilforoushzadeh M. A., Aghdami N. (2020). Maintaining hair inductivity in human dermal papilla cells: A review of effective methods. Skin. Pharmacol. Physiol. 33 (5), 280–292. 10.1159/000510152 [PubMed] [CrossRef] [Google Scholar]
- Tan K. H., Tan S. S., Ng M. J., Tey W. S., Sim W. K., Allen J. C., et al. (2017). Extracellular vesicles yield predictive pre-eclampsia biomarkers. J. Extracell. Vesicles 6 (1), 1408390. 10.1080/20013078.2017.1408390 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Tao S. C., Guo S. C., Li M., Ke Q. F., Guo Y. P., Zhang C. Q. (2017). Chitosan wound dressings incorporating exosomes derived from MicroRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl. Med. 6 (3), 736–747. 10.5966/sctm.2016-0275[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Thakur, A., Shah, D., Rai, D., Parra, D. C., Pathikonda, S., Kurilova, S., & Cili, A. (2023). Therapeutic Values of Exosomes in Cosmetics, Skin Care, Tissue Regeneration, and Dermatological Diseases. Cosmetics, 10(2), 65. https://doi.org/10.3390/cosmetics10020065
- Tkach M., Thery C. (2016). Communication by extracellular vesicles: Where we are and where we need to go. Cell. 164(6), 1226–1232. 10.1016/j.cell.2016.01.043 [PubMed] [CrossRef] [Google Scholar]
- Trounson A., McDonald C. (2015). Stem cell therapies in clinical trials: Progress and challenges. Cell. Stem Cell. 17 (1), 11–22. 10.1016/j.stem.2015.06.007 [PubMed] [CrossRef] [Google Scholar]
- van Niel G., D'Angelo G., Raposo G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 19 (4), 213–228. 10.1038/nrm.2017.125 [PubMed] [CrossRef] [Google Scholar]
- Vaswani K., Koh Y. Q., Almughlliq F. B., Peiris H. N., Mitchell M. D. (2017). A method for the isolation and enrichment of purified bovine milk exosomes. Reprod. Biol. 17 (4), 341–348. 10.1016/j.repbio.2017.09.007 [PubMed] [CrossRef] [Google Scholar]
- Villarroya-Beltri C., Baixauli F., Gutierrez-Vazquez C., Sanchez-Madrid F., Mittelbrunn M. (2014). Sorting it out: Regulation of exosome loading. Semin. Cancer Biol. 28, 3–13. 10.1016/j.semcancer.2014.04.009 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Wang C., Wang M., Xu T., Zhang X., Lin C., Gao W., et al. (2019a). Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 9 (1), 65–76. 10.7150/thno.29766 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wang L., Hu L., Zhou X., Xiong Z., Zhang C., Shehada H. M. A., et al. (2017). Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 7 (1), 13321. 10.1038/s41598-017-12919-x [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wang M., Wang C., Chen M., Xi Y., Cheng W., Mao C., et al. (2019b). Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano 13 (9), 10279–10293. 10.1021/acsnano.9b03656 [PubMed] [CrossRef] [Google Scholar]
- Wang X., Gu H., Huang W., Peng J., Li Y., Yang L., et al. (2016). Hsp20-Mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabetes 65 (10), 3111–3128. 10.2337/db15-1563 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wang X. Y., Guan X. H., Yu Z. P., Wu J., Huang Q. M., Deng K. Y., et al. (2021). Human amniotic stem cells-derived exosmal miR-181a-5p and miR-199a inhibit melanogenesis and promote melanosome degradation in skin hyperpigmentation, respectively. Stem Cell. Res. Ther. 12 (1), 501. 10.1186/s13287-021-02570-9 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Wang Z., Popowski K. D., Zhu D., de Juan Abad B. L., Wang X., Liu M., et al. (2022). Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat. Biomed. Eng. 6, 791–805. 10.1038/s41551-022-00902-5 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Willms E., Cabanas C., Mager I., Wood M. J. A., Vader P. (2018). Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 9, 738. 10.3389/fimmu.2018.00738[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Witwer K. W., Buzás E. I., Bemis L. T., Bora A., Lässer C., Lötvall J., et al. (2013). Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2, 20360. 10.3402/jev.v2i0.20360[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Xiong M., Zhang Q., Hu W., Zhao C., Lv W., Yi Y., et al. (2021). The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol. Res. 166, 105490. 10.1016/j.phrs.2021.105490 [PubMed] [CrossRef] [Google Scholar]
- Xu J., Bai S., Cao Y., Liu L., Fang Y., Du J., et al. (2020). <p>miRNA-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice</p>. Diabetes Metab. Syndr. Obes. 13, 1259–1270. 10.2147/DMSO.S243549 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Yan D., Li S. H., Zhang A. L., Xiao Y., Huang Z. C. (2021). A clinical study of platelet-rich fibrin combined with autologous high-density fat transplantation in augmentation rhinoplasty. Ear Nose Throat J. 2021, 1455613211016902. 10.1177/01455613211016902 [PubMed] [CrossRef] [Google Scholar]
- Yang G., Chen G., Gu Z. (2021). Transdermal drug delivery for hair regrowth. Mol. Pharm. 18 (2), 483–490. 10.1021/acs.molpharmaceut.0c00041 [PubMed] [CrossRef] [Google Scholar]
- Yang G., Chen Q., Wen D., Chen Z., Wang J., Chen G., et al. (2019a). A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano 13 (4), 4354–4360. 10.1021/acsnano.8b09573 [PubMed] [CrossRef] [Google Scholar]
- Yang J. S., Lee J. C., Byeon S. K., Rha K. H., Moon M. H. (2017). Size dependent lipidomic analysis of urinary exosomes from patients with prostate cancer by flow field-flow fractionation and nanoflow liquid chromatography-tandem mass spectrometry. Anal. Chem. 89 (4), 2488–2496. 10.1021/acs.analchem.6b04634 [PubMed] [CrossRef] [Google Scholar]
- Yang X. X., Sun C., Wang L., Guo X. L. (2019b). New insight into isolation, identification techniques and medical applications of exosomes. J. Control. Release 308, 119–129. 10.1016/j.jconrel.2019.07.021 [PubMed] [CrossRef] [Google Scholar]
- Yari H., Mikhailova M. V., Mardasi M., Jafarzadehgharehziaaddin M., Shahrokh S., Thangavelu L., et al. (2022). Emerging role of mesenchymal stromal cells (MSCs)-derived exosome in neurodegeneration-associated conditions: A groundbreaking cell-free approach. Stem Cell. Res. Ther. 13 (1), 423. 10.1186/s13287-022-03122-5 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Yeh Y. Y., Ozer H. G., Lehman A. M., Maddocks K., Yu L., Johnson A. J., et al. (2015). Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of BCR signaling. Blood 125 (21), 3297–3305. 10.1182/blood-2014-12-618470 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Yi Y. W., Lee J. H., Kim S. Y., Pack C. G., Ha D. H., Park S. R., et al. (2020). Advances in analysis of biodistribution of exosomes by molecular imaging. Int. J. Mol. Sci. 21 (2), 665. 10.3390/ijms21020665 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Yoshida M., Satoh A., Lin J. B., Mills K. F., Sasaki Y., Rensing N., et al. (2019). Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell. Metab. 30 (2), 329–342.e5. 10.1016/j.cmet.2019.05.015 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Yuan R., Dai X., Li Y., Li C., Liu L. (2021). Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol. Med. Rep. 24 (5), 758. 10.3892/mmr.2021.12398 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zeng L., Wang H., Shi W., Chen L., Chen T., Chen G., et al. (2021). Aloe derived nanovesicle as a functional carrier for indocyanine green encapsulation and phototherapy. J. Nanobiotechnology 19 (1), 439. 10.1186/s12951-021-01195-7[PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhai M., Zhu Y., Yang M., Mao C. (2020). Human mesenchymal stem cell derived exosomes enhance cell-free bone regeneration by altering their miRNAs profiles. Adv. Sci. (Weinh). 7 (19), 2001334. 10.1002/advs.202001334 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhang B., Shi Y., Gong A., Pan Z., Shi H., Yang H., et al. (2016a). HucMSC exosome-delivered 14-3-3ζ orchestrates self-control of the Wnt response via modulation of YAP during cutaneous regeneration. Stem Cells 34 (10), 2485–2500. 10.1002/stem.2432 [PubMed] [CrossRef] [Google Scholar]
- Zhang J., Chen C., Hu B., Niu X., Liu X., Zhang G., et al. (2016b). Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through erk1/2 signaling. Int. J. Biol. Sci. 12 (12), 1472–1487. 10.7150/ijbs.15514 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhang J., Guan J., Niu X., Hu G., Guo S., Li Q., et al. (2015a). Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med.13, 49. 10.1186/s12967-015-0417-0 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhang K., Yu L., Li F. R., Li X., Wang Z., Zou X., et al. (2020a). <p>Topical application of exosomes derived from human umbilical cord mesenchymal stem cells in combination with sponge spicules for treatment of photoaging</p>. Int. J. Nanomedicine 15, 2859–2872. 10.2147/ijn.S249751 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhang X. G., Wang Y. H., Han C., Hu S., Wang L. Q., Hu J. H. (2015b). Effects of trehalose supplementation on cell viability and oxidative stress variables in frozen-thawed bovine calf testicular tissue. Cryobiology 70 (3), 246–252. 10.1016/j.cryobiol.2015.03.004 [PubMed] [CrossRef] [Google Scholar]
- Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. (2020b). <p>Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications</p>. Int. J. Nanomedicine 15, 6917–6934. 10.2147/IJN.S264498 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhang Y., Su J., Ma K., Li H., Fu X., Zhang C. (2022). Photobiomodulation promotes hair regeneration in injured skin by enhancing migration and exosome secretion of dermal papilla cells. Wound Repair Regen. 30 (2), 245–257. 10.1111/wrr.12989 [PubMed] [CrossRef] [Google Scholar]
- Zhao B., Li X., Shi X., Shi X., Zhang W., Wu G., et al. (2018). Exosomal MicroRNAs derived from human amniotic epithelial cells accelerate wound healing by promoting the proliferation and migration of fibroblasts. Stem Cells Int. 2018, 1–10. 10.1155/2018/5420463 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhou L., Wang H., Jing J., Yu L., Wu X., Lu Z. (2018). Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem. Biophys. Res. Commun. 500 (2), 325–332. 10.1016/j.bbrc.2018.04.067 [PubMed] [CrossRef] [Google Scholar]
- Zifkos K., Dubois C., Schafer K. (2021). Extracellular vesicles and thrombosis: Update on the clinical and experimental evidence. Int. J. Mol. Sci. 22 (17), 9317. 10.3390/ijms22179317 [PMC free article] [PubMed] [CrossRef] [Google Scholar
